SALURBAL - FAIR Principles
A primer on FAIR principles and the SALURBAL project.
The why
The problem with the current data ecosystem is that the outputs of millions of dollars of public research funder are stored within each recipient or research groups system, without any standards for sharing their outputs or data generated. This issue is prevalent in health informatics as well where a large commercial EMR market provides a wide selection of disparate informatics platforms that don’t work well with each other.
This means there is a large pool of data that cannot be found, accessed, interoperate with each other and thus unable to be reused. This problem is prevalent within institutions as well as on a industry/national/global level (COVID-19 is a prime example of how important and beneficial quick and reliable data sharing and integration is to epidemiological surveillanceand evidence based policy decisions).
FAIR Principles
Largely to address these data challenges, a collective of pharma, academia, government and publishing representatives published the FAIR data principles were published in 2016 (Wilkinson et al. 2016) to provide guiding principles for improved Findability, Accessibility, Interoperability, and Reuse of digital assets.
The principles emphasise machine-actionability (i.e., the capacity of computational systems to find, access, interoperate, and reuse data with none or minimal human intervention) because humans increasingly rely on computational support to deal with data as a result of the increase in volume, complexity, and creation speed of data.
FAIR data principles and direct value generated by FAIR data are displayed below (Wise et al. 2019).
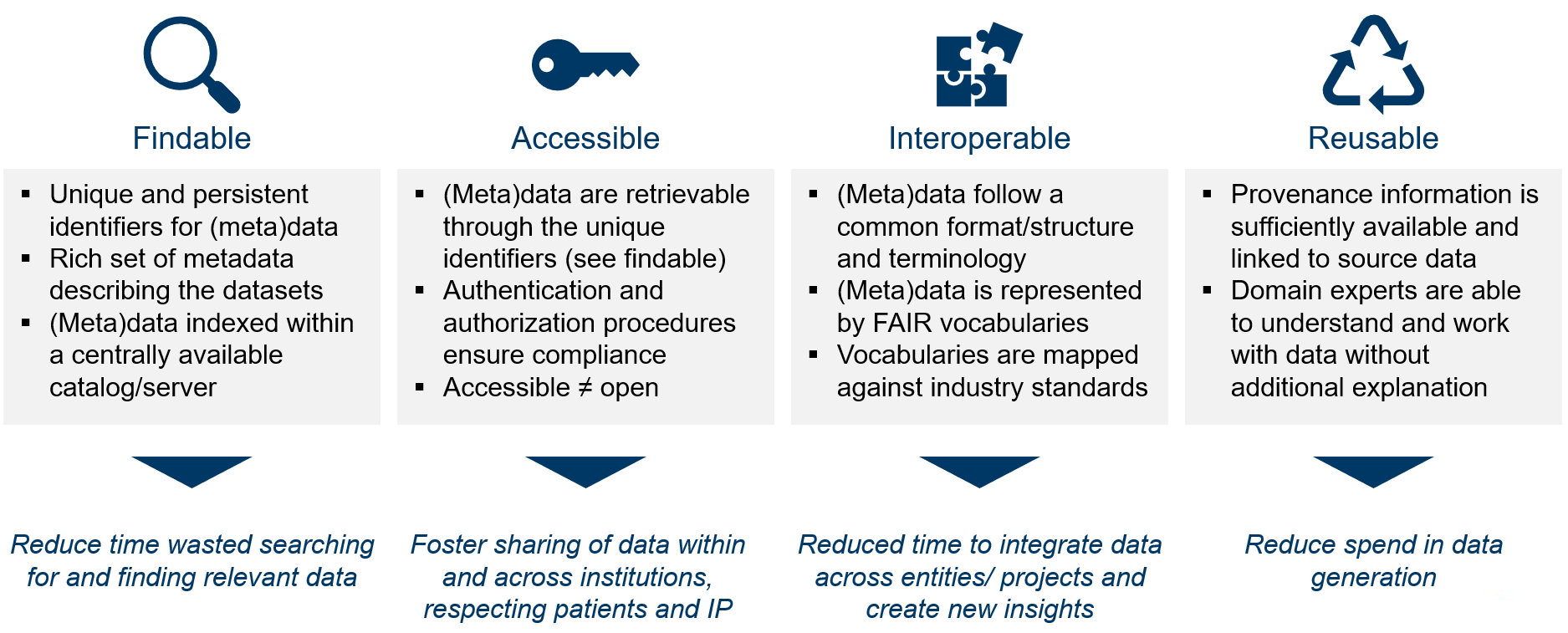
FAIR ≠ Open data
Open data (everyone share your data) does not mean the data is FAIR (easily reusable). The open data movement (Murray-Rust 2008) predates FAIR data but it quickly became apparent that there was not much value to just exposing our data to the world if other not could find, access, interoperate or reuse.
FAIR data (easily findable/reusable) does not equal open data. FAIR data are just a set best practices for data management and stewardship; the Accessibility principle states that FAIR projects should respect any ethical legal or contractual restrictions (Wilkinson et al. 2016). Authentication and authorization mechanism should be in place to prevent access to sensitive/protected data. However, if possible metadata or information about the data should be made available so others can find the data and go through proper channels for access. Even if your data is not open, it is still best practice to enforce FAIR principles to not only maximize the value of your data within the project but also to provide details.
Projects should aim to FAIR (regardless of whether it is open) in order to maximize the value of their data assets.
How to be FAIR?
Since the introduction of FAIR data principles in 2016, there is still no global multi-disiplenary accepted implementation for FAIR principles. However there have been FAIR implementations executed at several levels from within a project, to within a field/industry, to national or even global efforts. Current effort led by the go FAIR initiative seeks to profile the diverse FAIR implementation ecosystem in order to document convergent implementation methods (Schultes et al. 2020).
What does this mean for SALURBAL?
The first step is to achieve FAIR/machine-actionable data within our own project (SALURBAL). Then we can utilize some FAIR data services (e.g. ICPSR) to share our resource with the larger global FAIR data ecosystem.
Here we first evaluate the baseline SALURBAL infrastructure in terms of some FAIR principles. Before talking about our efforts to improve FAIRness of our project.